Bracco Diagnostics' MultiHance Contrast Agent Earns Expanded Labeling for Pediatric MRI
Bracco Diagnostics Inc. announced the labeling of its contrast agent MultiHance has obtained U.S. Food and Drug Administration (FDA) approval for an extension to include magnetic resonance imaging (MRI) of the central nervous system (CNS) in pediatric patients younger than 2 years of age (including term neonates). The agent may now be used in this patient population to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues.

Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging
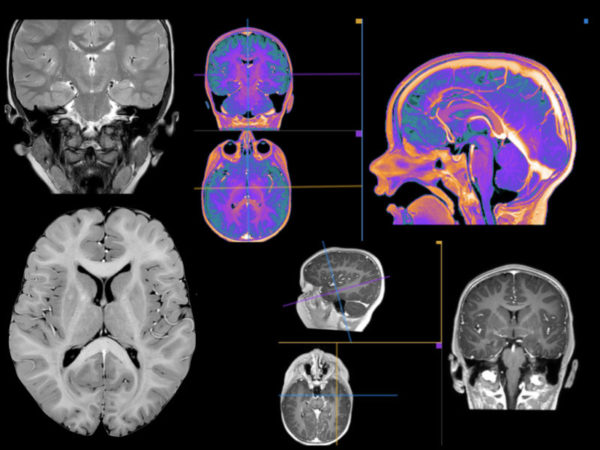
Episode 8: Gadolinium-Based Contrast Agents (GBCAs) Use in Pediatric MRI
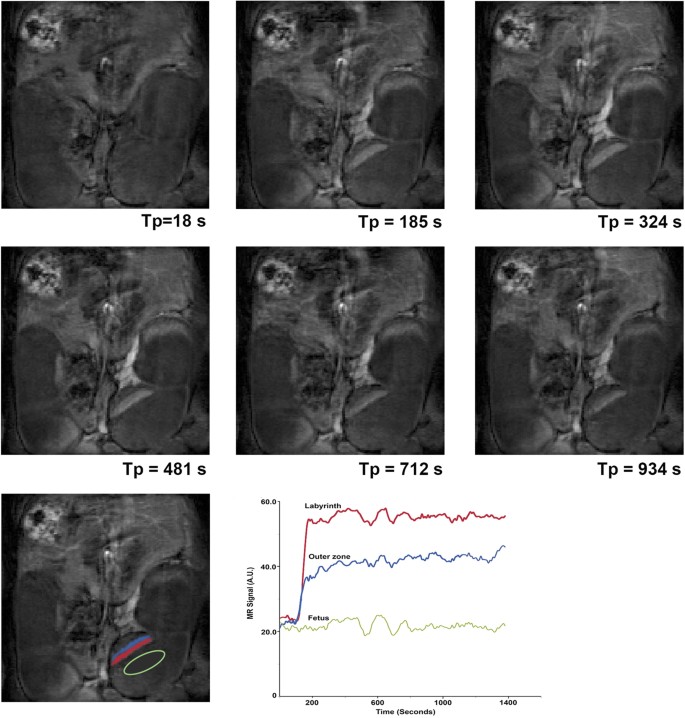
A liposomal Gd contrast agent does not cross the mouse placental barrier

Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers
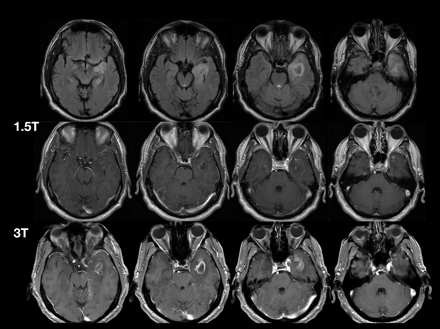
MR Imaging of Neoplastic Central Nervous System Lesions: Review and Recommendations for Current Practice

A liposomal Gd contrast agent does not cross the mouse placental barrier
PDF) 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives

Polymeric Metal Contrast Agents for T1-Weighted Magnetic Resonance Imaging of the Brain

Imaging Technology News - Magnetic Resonance Imaging (MRI)

Pediatric magnetic resonance angiography: to contrast or not to contrast

Amidst Increase in MRI Procedures, New Survey Finds 55% of Radiologists Have Concerns About Contrast Agent Availability

Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates