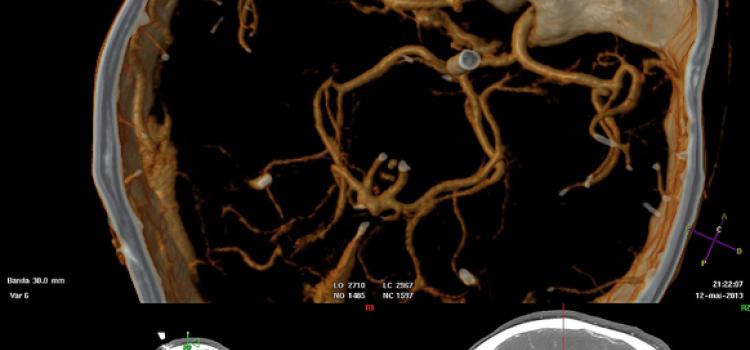
FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.

The Power In Prefilled Syringes
Contrast Media Imaging Technology News - 阿根廷vs乌拉圭直播

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
ACIST Features Contrast Injectors, Software

Short-, Mid-, and Long-term Strategies to Manage the Shortage of Iohexol

EHR Interventions for Contrast Media Shortage Impact CT Utilization

Siemens Healthineers Announces FDA Clearance of ARTIS icono ceiling Angiography System

Efficacy and Safety of Gadopiclenol with Contrast-enhanced MRI of the Central Nervous System Published

Contrast Media Injectors

Imaging Biometrics Submits FDA 510(k) Application for IB Zero G

SEC Filing GE HealthCare
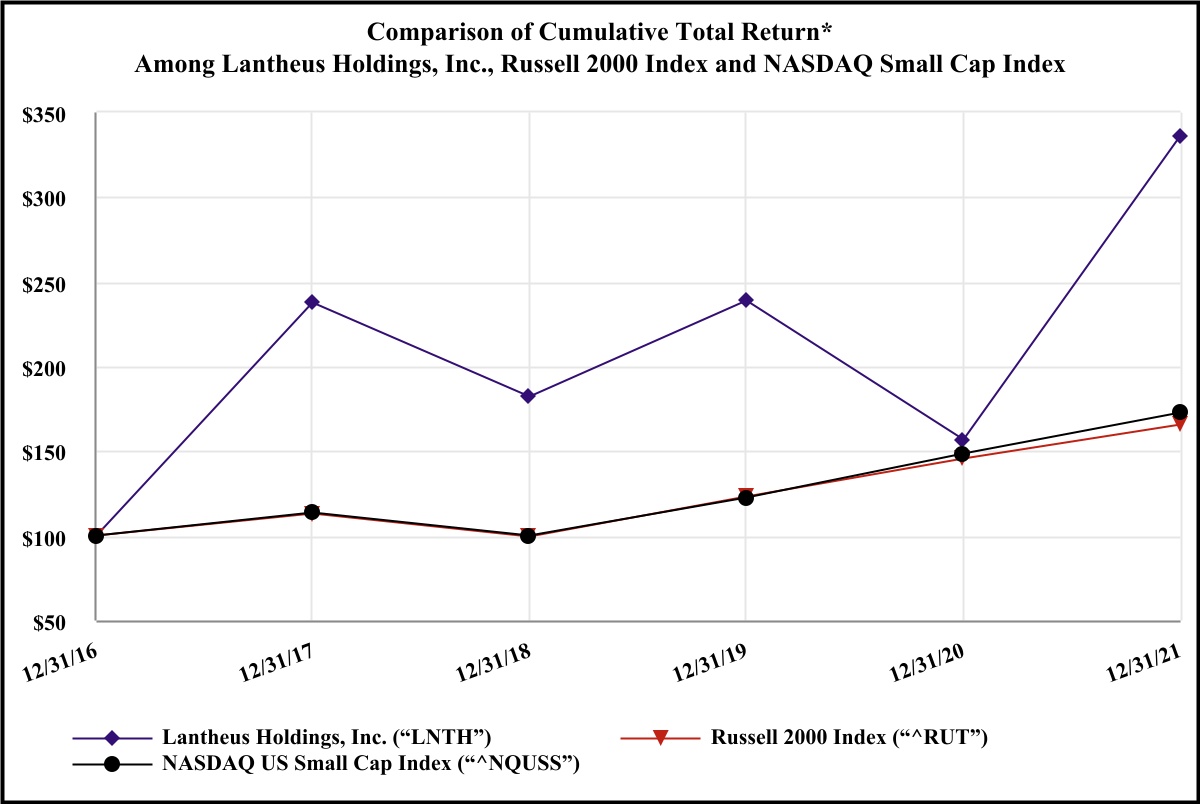
lnth-20211231

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages

FDA to Allow Bracco to Import Iomeron Iodinated Contrast Media as Shortage Drags On
articles • APPLIED RADIOLOGY