
Boyer Recalls Six Brands of Sodium and Potassium Hydroxide Due to Failure to Meet Child-Resistant Packaging Requirement; Injuries Reported
Consumers should immediately store the recalled products in a safe location out of reach of children and contact Boyer for a free replacement child-resistant cap.

PDF) 食品化学Food Chemistry(2007)
blocktrail-webwallet/src/lib/zxcvbn/dist/zxcvbn.js.map at master · blocktrail/blocktrail-webwallet · GitHub

Boyer Recalls Soap-Making Chemicals After Child Suffers Burn Injuries

Boyer Recalls Six Brands of Sodium and Potassium Hydroxide Due to Failure to Meet Child-Resistant Packaging Requirement; Injuries Reported

Persistence of microbiological hazards in food and feed production and processing environments - - 2024 - EFSA Journal - Wiley Online Library

CPSC Warns Consumers to Immediately Stop Using Mollys Products' Sodium Hydroxide Due to Risk of Chemical Burns and Irritation to the Skin and Eyes, and Failure to Meet Child-Resistant Packaging Requirements; Sold

Is It Safe to Make Soap at Home?

Pro Supply Outlet Recalls Sodium and Potassium Hydroxide Products Due to Failure to Meet Child-Resistant Packaging Requirement and Violation of FHSA Labeling Requirement (Recall Alert)

Children's Product Safety

Boyer Recalls Six Brands of Sodium and Potassium Hydroxide Due to Failure to Meet Child-Resistant Packaging Requirement; Injuries Reported

Food Safety Ian C. Shaw The Science of Keeping Food Safe by carolinafaraujo - Issuu

1981-82_v04,n08_Imprint by Editor Imprint - Issuu
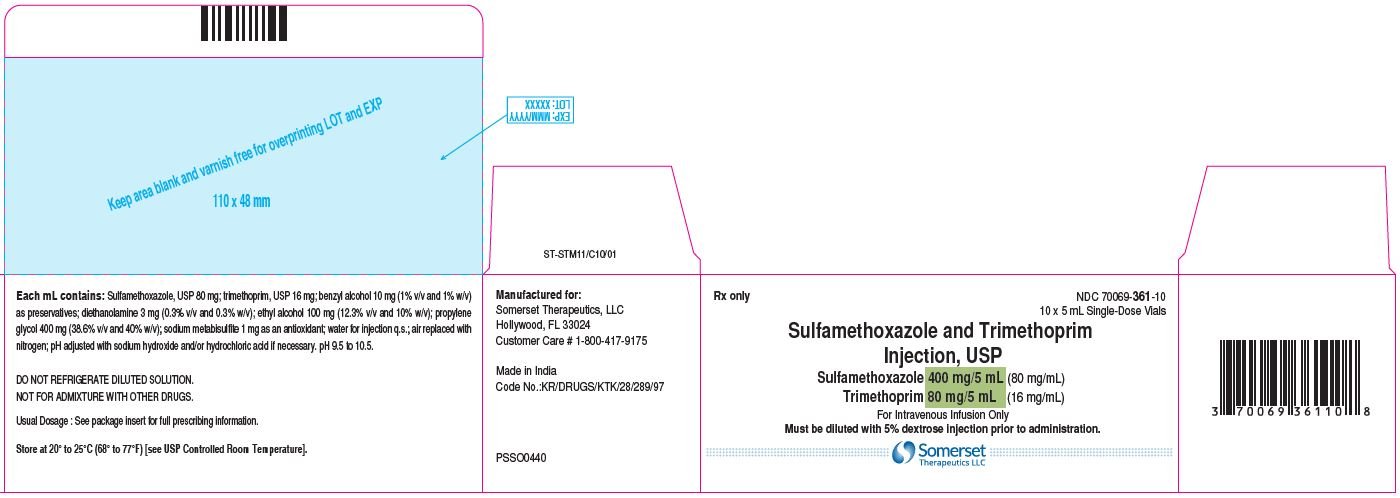
Sulfamethoxazole and Trimethoprim Injection: PI

biOrigins Sodium Hydroxide Products Recalled Due to Failure to Meet Child-Resistant Packaging Requirement and Violation of FHSA Labeling Requirement; Imported by Madar Corporation; Sold Exclusively at .com (Recall Alert)









