FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.

Home Imaging Technology News - 阿根廷vs乌拉圭直播

Consorta Extends Two Kodak Contracts
Angiography Imaging Technology News

Federal Register :: Authorization of Emergency Use of an In Vitro Diagnostic Device for Detection of Monkeypox Virus; Availability

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages

Toshiba Demonstrates Angiography Dose Management, Procedural Guidance

Ultravist (Iopromide) Paves New Path in Breast Cancer Detection - Xtalks

Growth in Cerebral Aneurysms Increases Risk of Rupture
IOMERON- iomeprol injection injection, solution
articles • APPLIED RADIOLOGY
ACIST Features Contrast Injectors, Software

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron® (iomeprol injection) to Address Supply Shortages
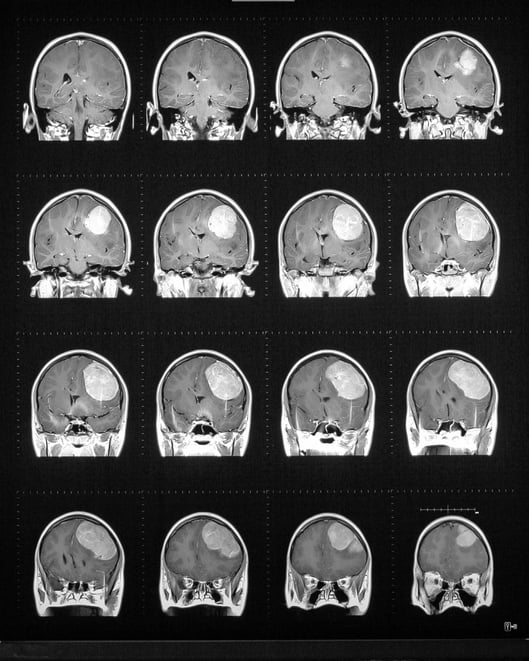
Guerbet Announces Commercial Launch and First Patient Dosing of Elucirem (Gadopiclenol) Injection









:max_bytes(150000):strip_icc()/galentines-day-gifts-2000-17fc77cd936a469aae4914acba9e3deb.jpg)