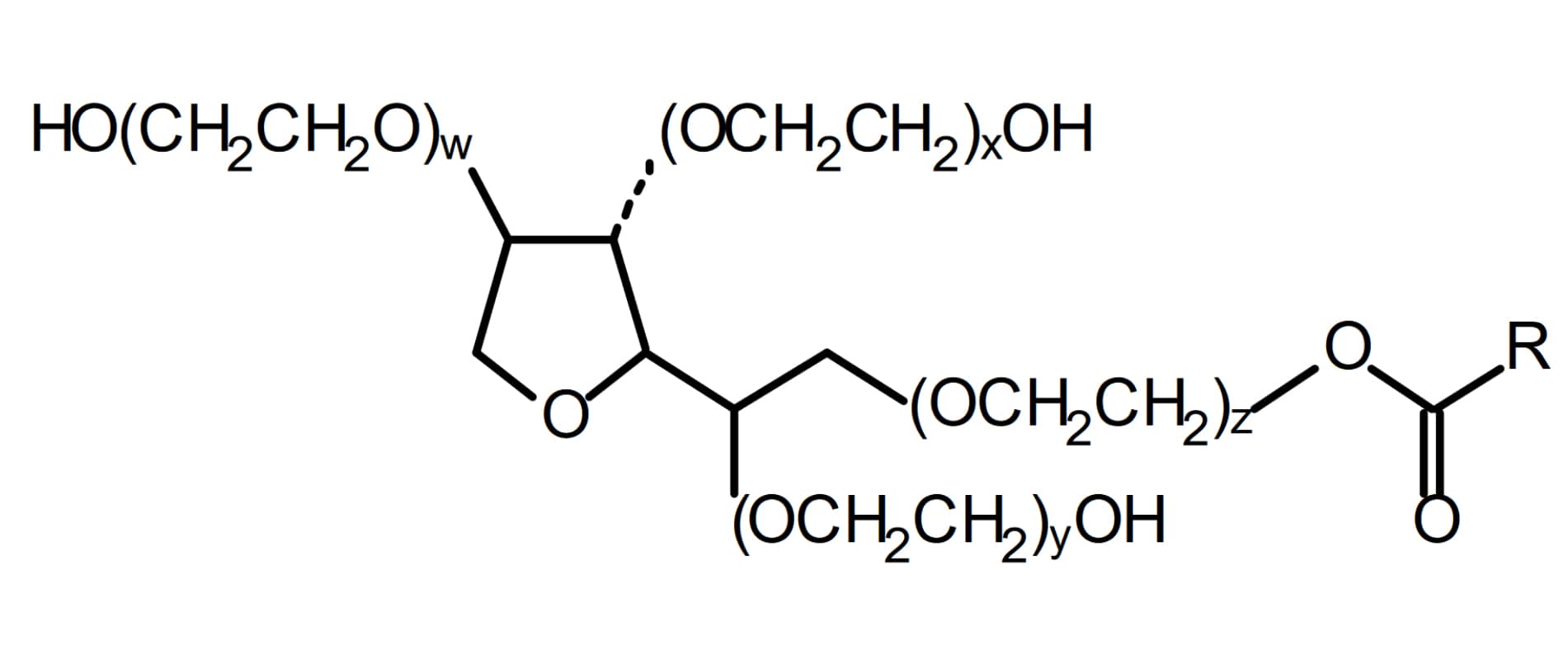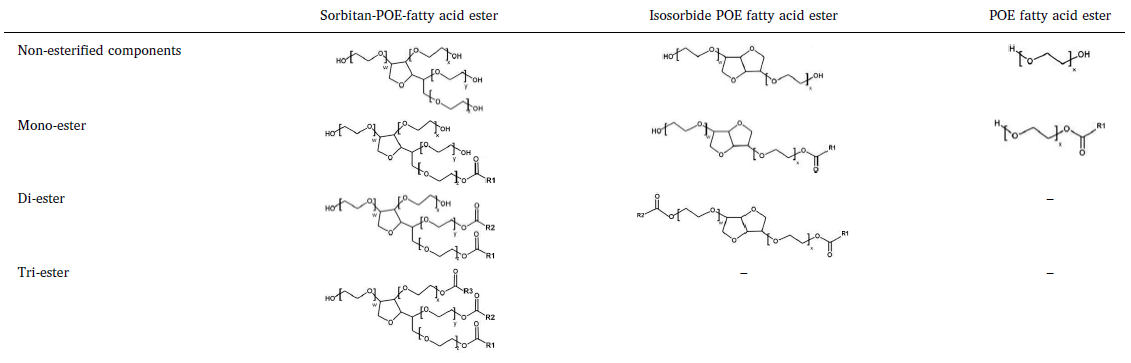
Development and validation of a selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations using UPLC QDa detection - Pharma Excipients
Polysorbates are widely used as non-ionic surfactant in biopharmaceutical formulations. Recently, the degradation of polysorbate moved into the focus of
Polysorbates are widely used as non-ionic surfactant in biopharmaceutical formulations. Recently, the degradation of polysorbate moved into the focus of attention, because in several published studies it was described, that stability issues in polyso

All-in-one stability indicating polysorbate 20 degradation root-cause analytics via UPLC-QDa - ScienceDirect

Discrimination of Polysorbate 20 by High-Performance Liquid Chromatography-Charged Aerosol Detection and Characterization for Components by Expanding Compound Database and Library - ScienceDirect
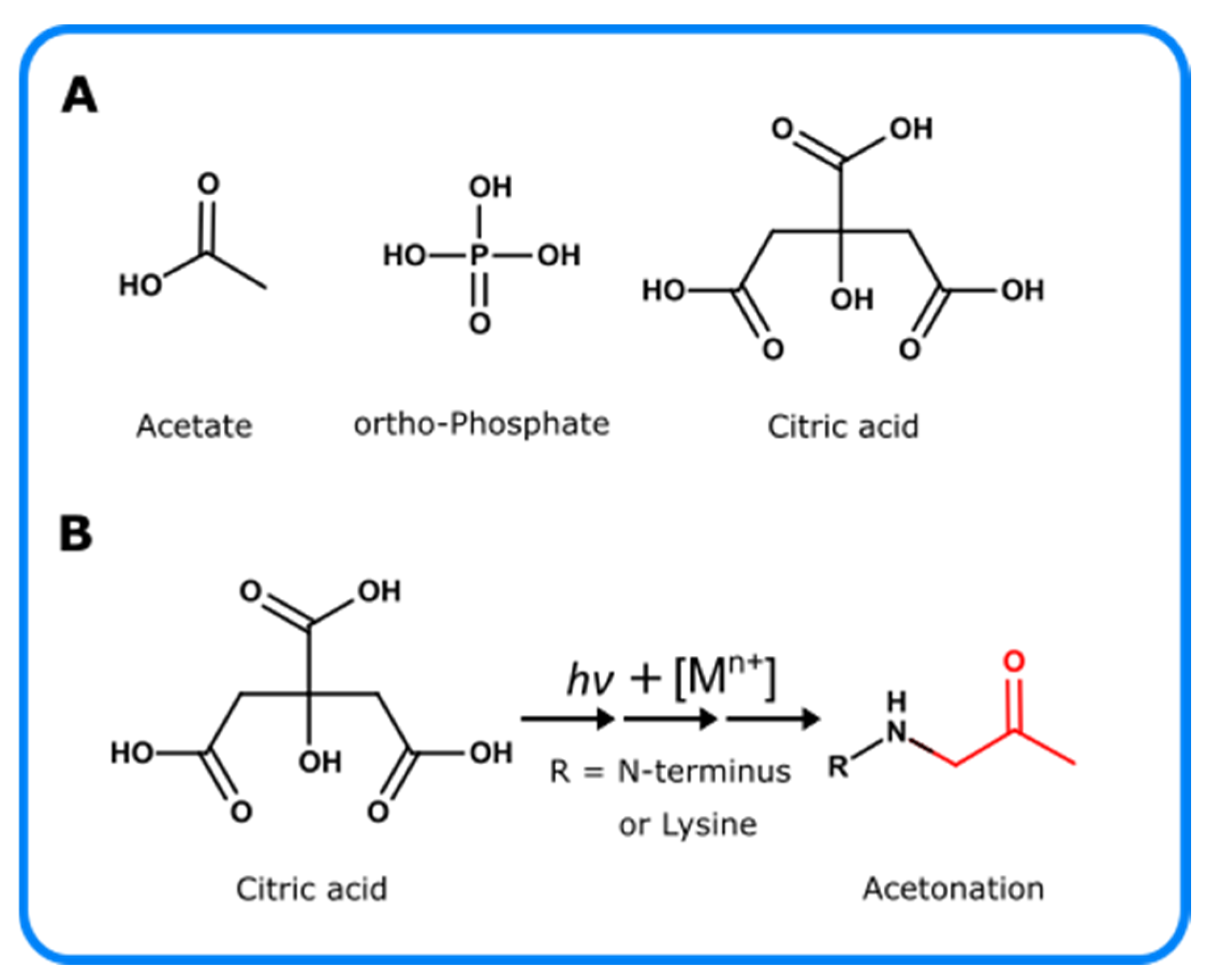
Pharmaceutics, Free Full-Text

Complex Micellization Behavior of the Polysorbates Tween 20 and Tween 80
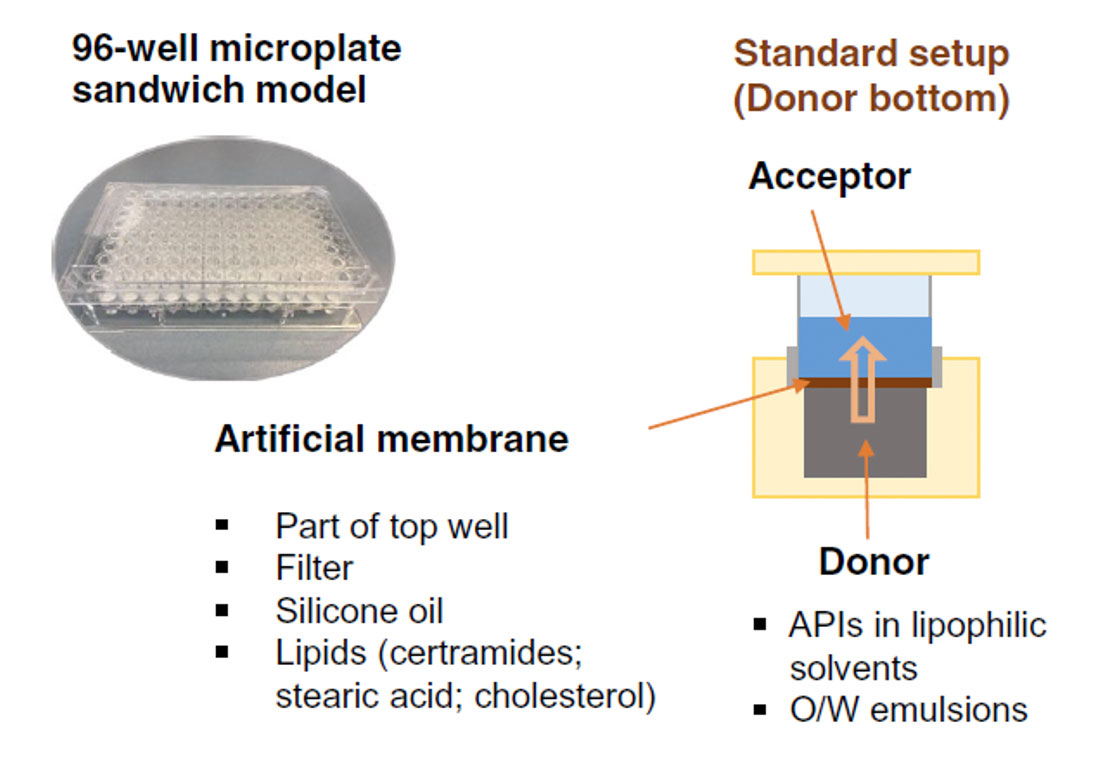
RaDes EN Archive - RaDes GmbH

Industry Perspective on the Use and Characterization of Polysorbates for Biopharmaceutical Products Part 2: Survey Report on Control Strategy Preparing for the Future - Journal of Pharmaceutical Sciences

All-in-one stability indicating polysorbate 20 degradation root-cause analytics via UPLC-QDa - ScienceDirect

DDL2019 Digital Proceedings by info-ddl-conference - Issuu

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism

PDF) Comparative Stability Study of Polysorbate 20 and Polysorbate 80 Related to Oxidative Degradation
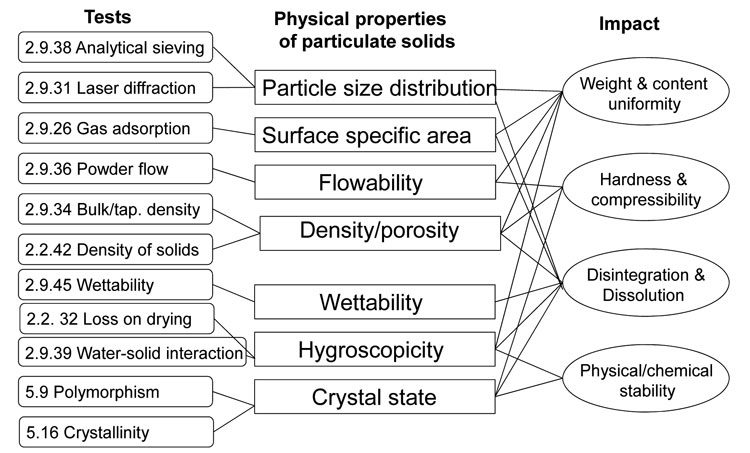
The central role of excipients in drug formulation - Pharma Excipients